· 10/08/2010
Retinal vein occlusions.

· 26/03/2013
As-needed treatment with ranibizumab 0.5 mg in patients with neovascular age-related macular degeneration. (FALTA)

· 08/10/2010
A Review of Ranibizumab Clinical Trial Data in Exudative Age-Related Macular Degeneration and How to Translate It into Daily Practice.
· 03/01/2013
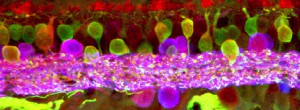
Optical Coherence Tomography Assessment of Apparent Foveal Swelling in Patients with Foveal Sparing Secondary to Geographic Atrophy.
· 26/03/2013
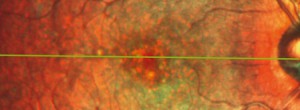
GAIN. Characterization of geographic atrophy progression in patients with age-related macular degeneration: evolution and risk factors associated with geographic atrophy progression

· 26/03/2013
Oral Omega 3 Drusen: A phase II randomized, double-mask study to establish the safety and efficacy of High-Dose Oral Omega 3 in subjects with high-risk drusen secondary to dry age-related macular degeneration
· 26/03/2013
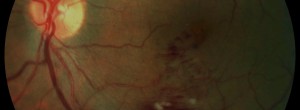
The Brighter Study: a 24-month, phase IIIB, open-label, randomized, active controlled, three-arm, multicenter study assessing the efficacy and safety of an individualized, stabilization criteria-driven pro re nata dosing regimen with 0.5-mg ranibizumab intravitreal injections applied as monotherapy or with adjunctive laser photocoagulation in comparison to laser photocoagulation in patients with visual impairment due to macular edema secondary to branch retinal vein occlusion (BRVO)
· 26/03/2013
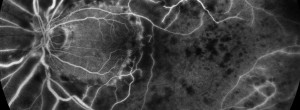
The Crystal Study: a 24-month, phase IIIB, open-label, single arm, multicenter study assessing the efficacy and safety of an individualized, stabilization criteria-driven pro re nata dosing regimen with 0.5-mg ranibizumab intravitreal injections applied as monotherapy in patients with visual impairment due to macular edema secondary to central retinal vein occlusion (CRVO)
· 26/03/2013
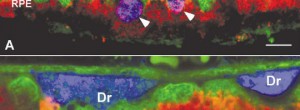
MPL4 DRUSEN. A phase I study to establish the safety and efficacy of retinal pigment epithelium micropulse laser in subjects with high-risk drusen secondary to age-related macular degeneration
· 26/03/2013
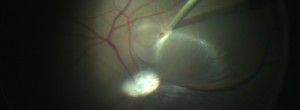
Translational research in regenerative stem cell therapies for retinal degenerative diseases: from the swine animal model of retinal atrophy to clinical trials in human patients with age-related macular degeneration, retinitis pigmentosa and Stargardt’s disease
· 26/03/2013
GAP. A phase I escalating dose of subretinal sodium iodate: a toxi-induced animal model of geography atrophy of RPE and phtotoreceptors

· 26/03/2013
Pilot phase II clinical trial to evalute safety of human fetal retinal transplant in the treatment of geographic atrophy secondary to age-related macular degeneration and retinitis pigmentosa
· 26/03/2013
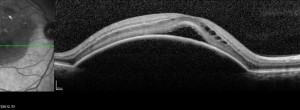
An open-label investigator research trial to study the safety and efficacy of a combination of a pro re nata regimen with fixed interval regimen intravitreal injections of ranibizumab in the treatment of choroidal neovascularization in subjects with neovascular age-related macular degeneration

· 26/03/2013
An open-label investigator research grant trial to establish the safety of intravitreous injections of E10030 (anti-PDGF pegylated aptamer) given in combination with Lucentis® in subjects with neovascular age-related macular degeneration
· 26/03/2013
A phase II, randomized, double-masked, controlled trial to establish the safety and efficacy of intravitreous injections of E10030 (anti-PDGF pegylated aptamer) given in combination with Lucentis® in subjects with neovascular age-related macular degeneration

