ما يميّزنا عن الآخرين؟
الطب 360º
في مركز "البقعة"، نؤمن بمفهوم الطب الشامل:
- نحن نركز على الشفاء
- نقدم طبا على مقاس الشخص
- نخصص لكل مريض الوقت الذي يحتاج إليه
- نبحث من أجل إيجاد علاجات جديدة
ونضيف إلى الجدية الطبية عدة خدمات من أجل العناية بالمريض بشكل جامل من خلال برنامج خدمات حضرية دون مصاريف إضافية.
- الرعاية النفسية
- إرشاد غذائي
- إرشاد بخصوص التقنيات المساعدة
- خدمات مرافقة
المرضى الدوليّون
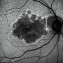
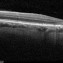
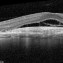
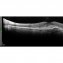
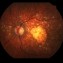
ساعدنا على وقف الأمراض البصرية التنكسية تقوم مؤسسة BMF بالبحث حول العلاجات الفعالة من أجل شفاء مزيد من حالات العمى. تعاون مع مؤسسة BMF برشلونة ماكولا فونديشن: البحث من أجل الرؤية