Clinical trial in antiretroviral-naive patients with choroidal neovascularization secondary to AMD 19/03/2014
Description
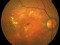
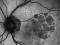
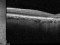
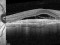
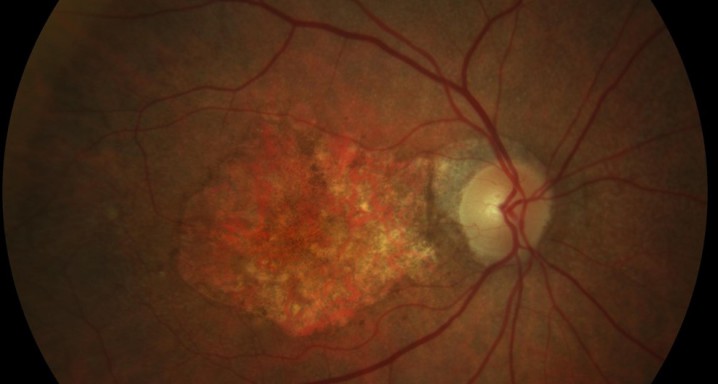
A phase 4, multi-centre, three-arm, randomised, open-label [SF1] clinical trial lasting 12 months, to evaluate the efficacy and safety of two treatment systems: the bimonthly or individualised flexible PRN “treat-and-extend” regimen; versus a PRN regimen of intravitreal injections of Ranibizumab 0.5 mg in keeping with stabilisation criteria based on monthly controls [SF2] in antiretroviral-naive patients with choroidal neovascularization secondary to age-related macular degeneration. An IN-EyE study.
If you are interested in taking part in a clinical trial, please fill in this form to contact us or call +34 935 950 155
Our team of professionals will answer you as soon as possible and we will assess whether you are eligible.
Author
Dr Jordi Monés, médecin, titulaire d’un doctorat
Inscrit à l'ordre des médecins de Barcelone, sous le numéro 22838
Directeur
Docteur en médecine et chirurgie
Spécialiste en ophtalmologie
Spécialiste de la rétine, de la macula et du vitré