GALE: evaluating the long-term safety and efficacy of intravitreal pegcetacoplan in patients with GA secondary to AMD 04/12/2022
Description
Its'a Phase III, open-label, multicenter clinical trial evaluating the long-term safety and efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
Goal
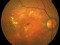
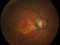
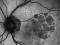
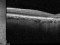
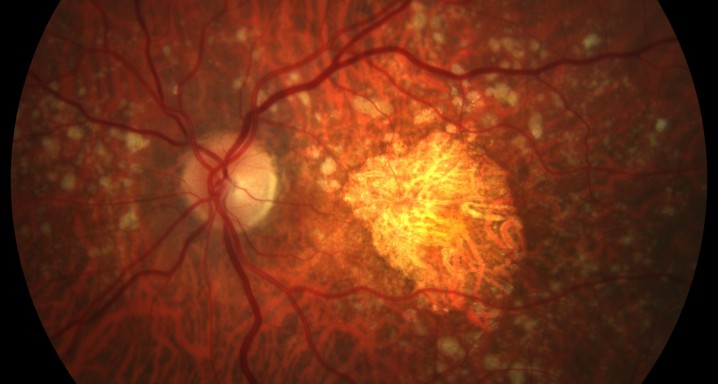
The main objective of this study is to evaluate the long-term safety of intravitreal pegcetacoplan, as well as the changes in the lesion measured by autofluorescence.
Drug
Biochemical, genetic and clinical lines of evidence in humans indicate that the complement system plays a role in the aetiology of AMD. Complement protein C3 (which has a pro-inflammatory action), membrane attack complex and complement factor H are present in the drusen and basal laminar deposits of patients with AMD. Pegcetacoplan is a drug for intravitreal use that blocks the action of C3. The main action is anti-inflammatory.
Inclusion criteria
This study only accepts patients who have participated in the APL2-103 study or have completed the 24-month treatment of the APL2-303 (DERBY) or APL2-304 (OAKS) studies.
Duration
The duration of the trial is three years.