LUCERNE clinical trial: efficacy and safety of FARICIMAB regarding aflibercept in the treatment of exudative age-related macular degeneration 09/04/2019
IF YOU WANT TO PARTICIPATE IN A CLINICAL TRIAL, SEND US YOUR DATA HERE AND I WILL EVALUATE IF YOU ARE ELIGIBLE
Description
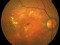
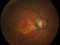
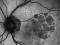
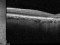
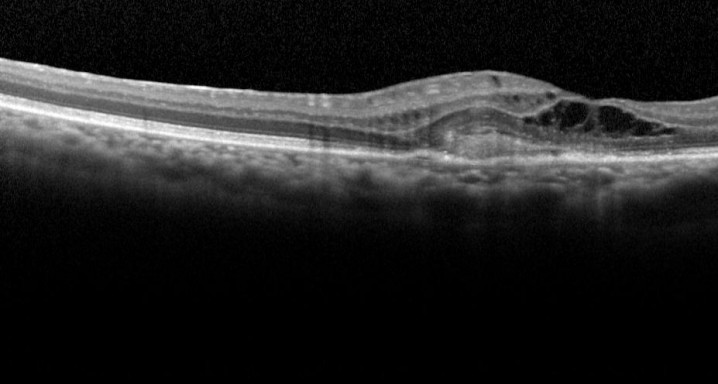
This is a multicentre, randomized and double-masking Clinical Trial Phase III of two parallel groups that evaluates the efficacy and safety of faricimab administered by intravitreal injeccions in patients with exudative age-related macular degenereation (AMD) compared to treatment with aflibercept (EYLEA®).
Main inclusion criteria
Patients over 50 years of exudative age-related macular degeneration who have not received study’s treatment for this eye disease
Visual acuity should be between 20/32 and 20/320.
Goal
The main objective is to demonstrate the efficacy of faricimab compared with aflibercept regarded the visual acuity result one year later
About the medicine
Faricimab is the first bispecific monoclonal antibody designed for intravitreal use that combines the antiangiogenic action of the medicine currently used for the AMD’s treatment with the anti-inflammatory action, neutralizing VEGF and angiopoietin-2 at the same time. The results in previous studies support the beginning of a Phase III trial to evaluate the efficacy and safety of faricimab in the treatment of neovascular AMD patients, in order to increase the intervals between treatments.
Duration
This essay lasts for two years.
IF YOU WANT TO PARTICIPATE IN A CLINICAL TRIAL, SEND US YOUR DATA HERE AND I WILL EVALUATE IF YOU ARE ELIGIBLE