Phase I/IIa study in subjects with Pigmentary Retinosis 18/11/2020
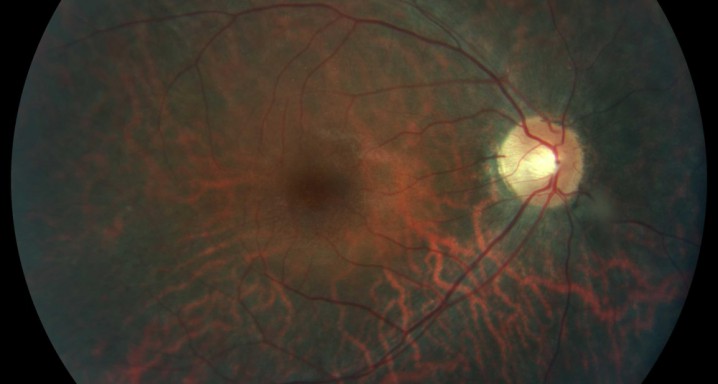
Description
A first, prospective, open, phase I/IIa human study of the safety and tolerability of human retinal progenitor cells (hRPC) transplanted into the subretinal space in subjects with Retinitis Pigmentosa (RP).
This is a prospective open phase I/II study to treat RP patients with transplanted human retinal progenitor cells. Participants will only be treated in one eye.
Main inclusion criteria
Adult men and women who are healthy (apart from their retinitis pigmentosa)
Patients’ visual acuity should be between 20/50 and 20/800 and be similar in both eyes
Aim
The aim of the study is to determine the safety and tolerability of human retinal progenitor cell transplantation in RP patients.
About the treatment
Subretinal stem cell injection in one eye. All the participants will receive the treatment and this will only be in one eye.
FREQUENTLY ASKED QUESTIONS
Who can participate in this clinical trial?
This clinical trial is aimed only at patients who have been diagnosed with RP and whose vision is between 20/50 and 20/800 and similar in both eyes.
What are the benefits and risks of participating in a trial?
Participation in a clinical trial offers a number of advantages for patients: they have the chance to access the most innovative treatments, with personalised and state-of-the-art care from medical experts. In addition, this is currently the only way to access new treatments that are not yet available to the general public.
Clinical trials are conducted according to strict ethical and scientific principles. At the Institute, we apply national and international standards and policies in order to protect the rights, safety and well-being of those who participate in them.
The risks of participation in a clinical trial may arise from the route of administration of the drug on the one hand and from the medication on the other. The risks of the former are better known; the treatment derivatives (in this case, the subretinal injection of human retinal progenitor cells) are partially known. In any case, the patient will be informed by the researcher of the possible adverse effects and discomforts resulting from their participation in the study, which may vary from one patient to another.
How much does it cost the patient?
Nothing
How long is the clinical trial?
The duration of this study is 24 months.
How many times do I need to go to the Institut de la Mácula?
After being selected to take part in this clinical trial, the patient must make 17 visits, which are scheduled according to the study protocol.