FOVISTA: Ensayo Clínico para pacientes con DMAE neovascular subfoveal 19/03/2014
Descripción
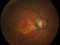
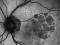
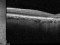
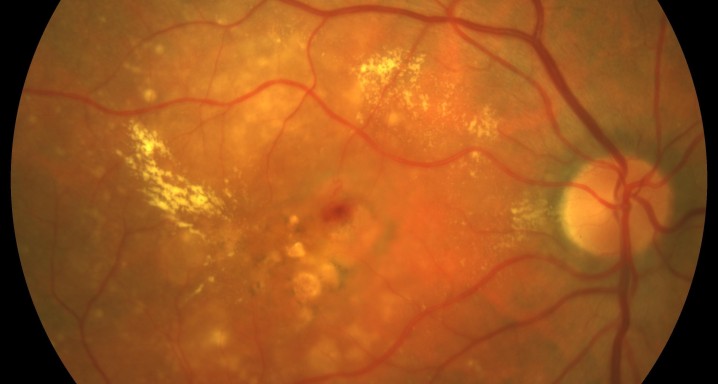
A phase 3 Randomized, doubled masked, controlled trial to establish the safety and efficacy of intravitreous administration of FOVISTA (anti-PDGF-b pegylated aptamer) administered in combination with Lucentis compared to Lucentis Monotherpahy in subjects with subfoveal neovascular age-related macular degeneration.
Objetivos
The objectives of the study are to evaluate the safety and efficacy of FOVISTA in combination with ranibizumab (Lucentis) as compared to ranibizumab monotherapy in patients with neovascular age-related macular degeneration who have not received previous treatment.
Más información sobre los ensayos clínicos en este vídeo.
Si quieres participar en un ensayo clínico consúltanos en el +34 93 595 01 55 y evaluaremos si eres elegible.
Inyecciones intravítreasDMAE exudativa o húmeda
Autor
Dr. Jordi Monés, M.D., Ph.D.
Número de Colegiado COMB: 22.838
Director
Doctor en Medicina y Cirugía
Especialista en Oftalmología
Especialista en Retina, Mácula y Vítreo