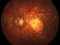
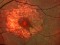
FOVISTA: Clinical Trial for subfoveal neovascular age-related macular degeneration 19/03/2014
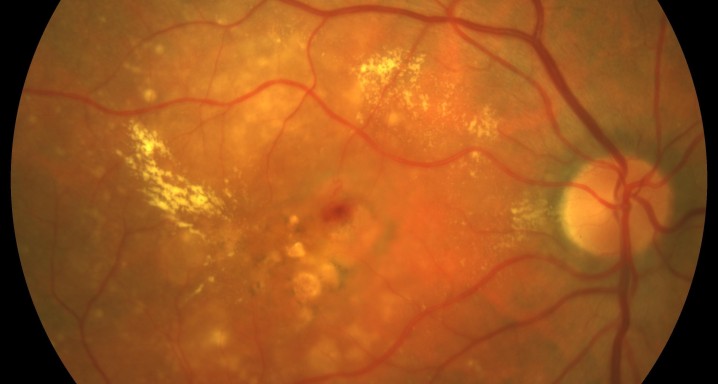
Description
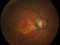
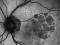
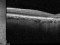
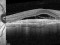
A phase 3 Randomized, doubled masked, controlled trial to establish the safety and efficacy of intravitreous administration of FOVISTA (anti-PDGF-b pegylated aptamer) administered in combination with Lucentis compared to Lucentis Monotherpahy in subjects with subfoveal neovascular age-related macular degeneration.
Objectives
The objectives of the study are to evaluate the safety and efficacy of FOVISTA in combination with ranibizumab (Lucentis) as compared to ranibizumab monotherapy in patients with neovascular age-related macular degeneration who have not received previous treatment.
If you are interested in taking part in a clinical trial, please fill in this form to contact us or call +34 935 950 155.
Our team of professionals will answer you as soon as possible and we will assess whether you are eligible.
Intravitreal injectionsExudative or wet AMD
Author
Dr. Jordi Monés, M.D., Ph.D.
COMB Medical license number: 22.838
Director
Doctor of Medicine and Surgery
Specialist in Ophthalmology
Specialist in Retina, Macula and Vitreorretinal