IONIS: safety and efficacy of multiple doses of IONIS-FB-LRX 04/12/2022
 Recruiting
Recruiting
Description
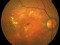
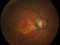
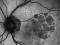
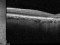
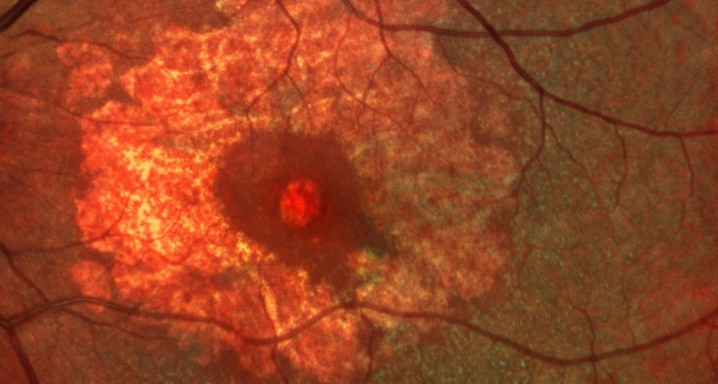
This is a randomized, double-masked, placebo-controlled phase II clinical trial to evaluate the safety and efficacy of multiple doses of IONIS-FB-LRX (also known as ISIS 696844), a Complement Factor B antisense oligonucleotide, in patients with geographic atrophy (GA) secondary to age-related macular degeneration (AMD).
Aim
- To evaluate the effect of ISIS 696844 on the rate of change of the area of atrophy of GA secondary to AMD as measured on fundus autofluorescence.
- To evaluate the effect of ISIS 696844 on plasmatic levels of Factor B and the serum activity of AH50 in patients with GA.
- To evaluate the effect of ISIS 696844 in the change of low luminance best-corrected visual acuity
Drug
The drug ISIS 696844 (40, 70 or 100 mg) or placebo will be administered by subcutaneous injection on weeks 1, 3 5 and every 4 weeks thereafter until week 45.
Inclusion criteria
- Men or women aged 50 years or older at the time of the informed consent
- Vaccination against Neisseria meningitidis (quadrivalent conjugate and serogroup B) and Streptococcus pneumoniae at least two weeks before first administration of the study drug
- A diagnosis of unilateral or bilateral GA confirmed by the Reading Center
- GA with a well-circumscribed area between 1.9 and 17 mm2
Duration
The study duration will be 18 months, including a screening period of 3 months, a treatment period of 12 months and a 3-month follow-up period.
It is possible that patients need to attend additional visits to control for potential adverse events or abnormal results along the course of the study. The frequency of this follow-up will be determined by the Medical Monitor in agreement with the Principal investigator.
Dry or atrophic AMD