VELODROME: efficacy, safety and pharmacokinetics of ranibizumab 100 mg/ml through a PDS in patients with nDMAE 04/12/2022
 Recruiting
Recruiting
Description
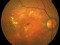
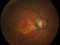
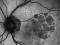
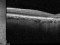
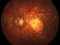
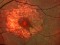
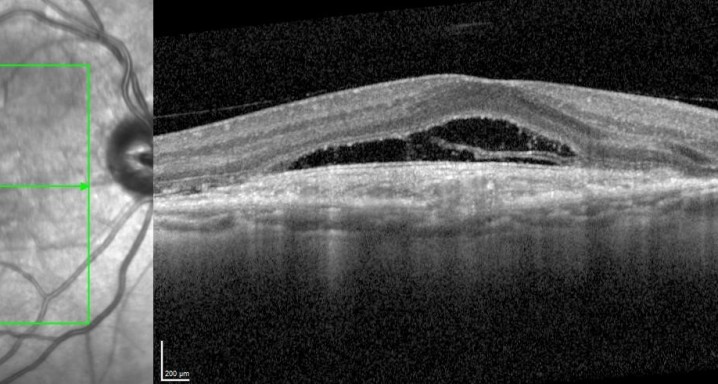
This study will evaluate the efficacy, safety and pharmacokinetics of ranibizumab 100 mg/ml released through a port-delivery system (PDS) will refill each 6 weeks as compared with a refill each 24 months in patients with neovascular age-related macular degeneration (nAMD).
Aim
The main objective is to evaluate the efficacy of ranibizumab 100 mg/ml delivered through a PDS system with refills at week 36 as compared to that at week 24.
Inclusion criteria
Patients older than 50 years diagnosed with nAMD in the 9 months prior to the selection visit. These patients need to receive a minimum of 3 intravitreal injections in the prior 6 months with proven response to therapy. If this last criterion is not met, the patient may enter a run-in phase where she/he will receive the minimum number of injections to met these requirements.
Duration
Between 24 and 36 weeks.
Dry or atrophic AMD