TALON (Extension): efficacy and safety of brolucizumab 6 mg in a maximum interval dosing schedule of 20 weeks for patients with nDMAE 04/12/2022
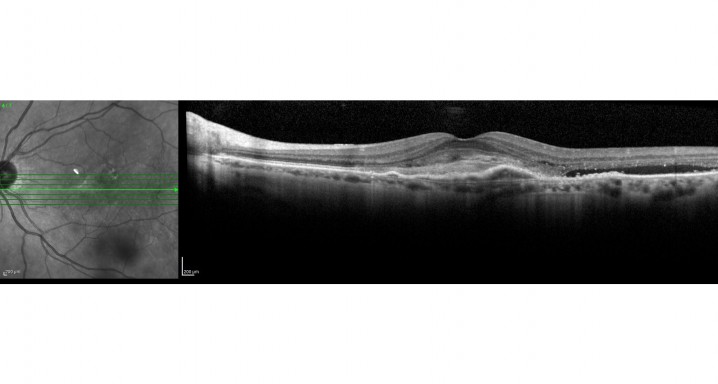
Description
A phase IIIb/IV open-label extension study with a single arm of 56 weeks to evaluate the efficacy and safety of brolucizumab 6 mg in a maximum interval dosing schedule of 20 weeks for the treatment of patients with neovascular age-related macular degeneration (nAMD) that completed the CRTH258A2303 (TALON) study.
Aims
- To evaluate the efficacy and safety of brolucizumab in patients with nAMD to evaluate the potential for achieving an interval of up to 20 weeks between intravitreal injections.
- To evaluate the durability of brolucizumab up to the week 56.
- To determine the functional effects of brolucizumab as measured on the change of best-corrected visual acuity between baseline and the mean of weeks 52 and 56.
Drug
Brolucizumab 6 mg/0,05 Ml.
Inclusion criteria
Participants who have successfully completed the TALON study up to week 64 (end of study visit).
Duration
56 weeks.